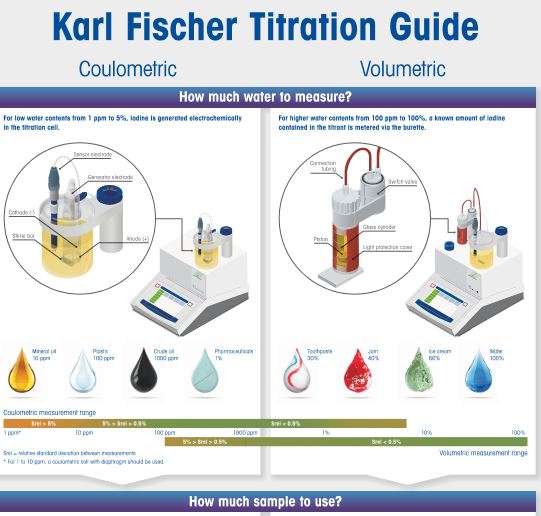
Karl Fischer Titration FAQs (Frequently Asked Questions)
Agnieszka Kossakowska (Honeywell Research Chemicals) and Michael Margreth (Metrohm) are KFT experts who share their knowledge and experience with users of this technique at seminars across the globe.
No matter where the technical training is performed, they often encounter the same questions. In an effort to help KFT users world over, Agnieszka & Michael have hereby compiled the most frequently asked questions and arranged these into categories for quick reference.
INSTRUMENT PREPARATION AND HANDLING
How can I check if the electrode is working correctly?
MM: We recommend carrying out a volumetric or coulometric Karl Fischer titration using a certified standard as a sample. In volumetry,
a threefold titer determination followed by a determination of a different standard is carried out and the recovery of the water content in the determination of the standard is calculated.
To check a coulometric system, a threefold determination with a certified water standard is carried out and the recovery calculated. If the recovery is between 97 and 103%, the system, including the electrode, is working fine.
The color of the working medium is an additional indicator whether the indication
is working properly. Pale yellow is perfect, whereas dark yellow or even pale brown suggests indication problems. If this happens, the indicator electrode should be cleaned.
How long can an electrode be stored in the KF reagent?
MM: Karl Fischer electrodes are made of glass and platinum. Therefore, the KF reagent does not affect the electrode. It can be s
How often should I change the drying agents?
AK: Each type of drying agent has a water adsorption capacity limit. How fast this limit
is reached depends on the humidity level in the laboratory – the higher the humidity, the more frequently the drying agent should be changed. Generally, this time should be weeks, not months. An average frequency is 4–6 weeks. Drying agents based on silica gel (e.g., HYDRANAL™-Humidity Absorber) offer the advantage of a color change when exhausted.
Can a molecular sieve be dried
and reused, or should it be replaced?
MM: Yes, of course, a molecular sieve can be dried and reused. We recommend drying for at least 24 hours at a temperature between 200 and 300°C.
Can I use HYDRANAL-Humidity Absorber instead of molecular sieves?
AK: Of course. It is perfect for drying carrier gases and on top of HYDRANAL-Composite one-component reagents. However, the choice of drying system depends on the size of the drying tube. HYDRANAL-Humidity Absorber beads are larger than molecular sieves, so they fit well into larger drying tubes. Any empty spaces inside the drying tube cause the air to pass between the drying agent beads, not through them.
Also, please note that these silica gel beads require a different regeneration procedure: they should be dried at 140°C until the color turns back.
How long does conditioning normally take?
MM: Conditioning of a freshly filled titration vessel normally takes around 2–4 minutes for volumetry, depending on the reaction speed (type of reagent), and around 15–30 minutes for coulometry. In combination with an oven, it might take a few minutes longer to reach
a stable drift due to the constant gas flow.
We recommend stabilizing the whole oven system for at least one hour.
Between single measurements in the same working medium, conditioning takes approximately 1–2 minutes. Take care that the original drift level is reached again.
When I start conditioning, there are a lot of bubbles in the coulometric cell for several minutes and a very high drift, even with fresh reagent. What could be the reason for this effect?
MM: At the anode, the generator electrode produces iodine from the iodide-containing reagent. The bubbles you see at the cathode are the result of the reduction of H+ ions
to hydrogen.
After opening the titration cell or filling it with fresh reagent, the conditioning step removes the water brought into the system and avoids falsifying the water content determination of the sample. Removing the water results in an increased drift level. During conditioning, the above-mentioned hydrogen is generated. The gas bubbles are therefore completely normal and not a cause for concern. Generally, the following rule applies: the more water in the titration vessel, the higher the drift value and the quantity of hydrogen formed.
How often should I clean the Karl Fischer equipment?
MM: There is no strict rule as to when you should clean the KF equipment. The cleaning intervals strongly depend on the type and
the amount of sample added to the titration cell. Poor solubility and contamination of
the indicator electrode (deposition layer on its surface) or memory effects due to large amounts of sample can be a reason for cleaning the equipment.
The drift can also be a good indicator. In case you observe higher and unstable drift values, I would recommend cleaning the titration cell or at least refilling the working medium.
DOWNLOAD THE FULL DOCUMENT HERE
Pursuing excellence
for more than 200 years
Honeywell's story began more than 200 years ago when German chemist Johann Daniel Riedel successfully manufactured pharmaceutical products. While the world has changed greatly since then, Honeywell remains committed to providing the highest quality chemicals through innovative and cutting-edge manufacturing processes. Explore Honeywell's diverse product line to discover how Greyhound Chromatography can help your lab.
Leading the way in innovation and safety since 1979, Hydranal™ reagents for Karl Fischer titration
have been recognized as the most trusted brand in the industry.
    |
    |
With more than 100 dedicated solvents and blends, Chromasolv™ solvents offer better impurity profiles and higher lot-to-lot consistency.
   |
Be confident and advance your research with Honeywell's Fluka™ Analytical Standards.
Honeywell's standards come complete with printed COA and free expert technical support.
 |
 |
You may also be interested in
An Overview of Honeywell's Research Chemicals
Honeywell Reference Materials Presentation
CONTACT US
Tel: +44 (0) 151 649 4000
Email: marketing@greyhoundchrom.com
FOLLOW US
YOU MAY ALSO BE INTERESTED IN OUR NEWSLETTER
About The Author
Susan Massie, Sales & Marketing Director, Greyhound Chromatography and Allied Chemicals Email: sue@greyhoundchrom.com
Susan Massie is the Sales & Marketing Director for Greyhound Chromatography and Allied Chemicals, affectionately known as 'Greyhound' in our scientific community. Greyhound was founded by Susan's husband Paul Massie almost 40 years ago, Susan hasn't been in the business for all of that time but has been involved with Greyhound for over 17 years. Greyhound continues to grow, expanding into new markets and taking on the challenges of our ever changing environment. It's heartwarming to witness the world waking up to the fact that we are damaging our planet on a daily basis. Every action we take has a direct effect on our planet and the world we leave behind for future generations. Susan is passionate about climate change and is happy to work in an industry that can have a direct effect on reducing the impact of our actions on the environment. All of the team at Greyhound take our responsibilities very seriously, the products that we supply are used by the world's leading scientists and chemists as they endeavour to monitor and repair the environment. All is not lost, if we all take responsibility for our actions, from reducing our waste and reusing or recycling our material collateral we can make a difference. The internet is full of useful advice and guidance, Susan is proud to contribute to that wealth of knowledge whenever she can.
Greyhound prides itself on personal service which provides prompt, efficient, cost-effective, safe delivery of all products. Greyhound provides technical advice and distribution of Certified Reference Standards and Materials, Laboratory Consumables, Solvents and Reagents across all scientific disciplines. Greyhound Chromatography offers over 1 Million products from its UK warehouse. The team at Greyhound are proud to support the work of the world's leading scientists and chemists as they challenge the abuse of our planet and try to make a difference to the world we leave behind for our ancestors.
You can view Susan's Linked In Profile here https://www.linkedin.com/in/susan-massie-79ab4121/